Boyle's Law Calculator
This Boyle's Law Calculator tool will calculate any parameter from the equation defined by Boyle's law PV = K or P1 × V1 = P2 × V2
To Calculate :
Pressure 1 :
Volume 1 :
Pressure 2 :
Volume 2 :
Result
x
If you use this great tool then please comment and/or like this page.
Average Rating: Tool Views: 419
Average Rating: Tool Views: 419
Subscribe for Latest Tools
How to use this Boyle's Law Calculator Tool?
How to use Yttags's Boyle's Law Calculator?
- Step 1: Select the Tool

- Step 2: Enter Following Options And Click On Calculate Button
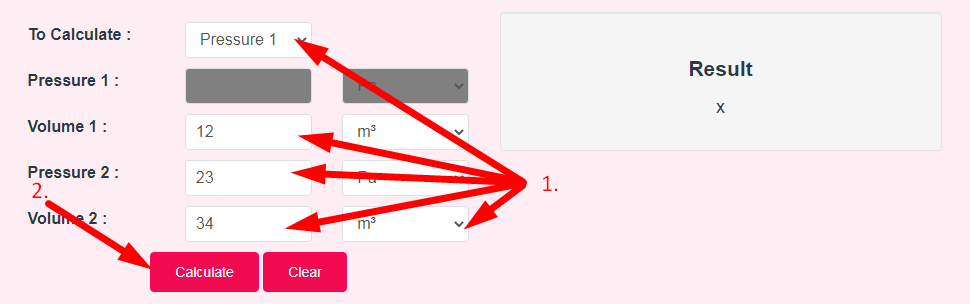
- Step 3: Check Your Boyle's Law Calculator Result
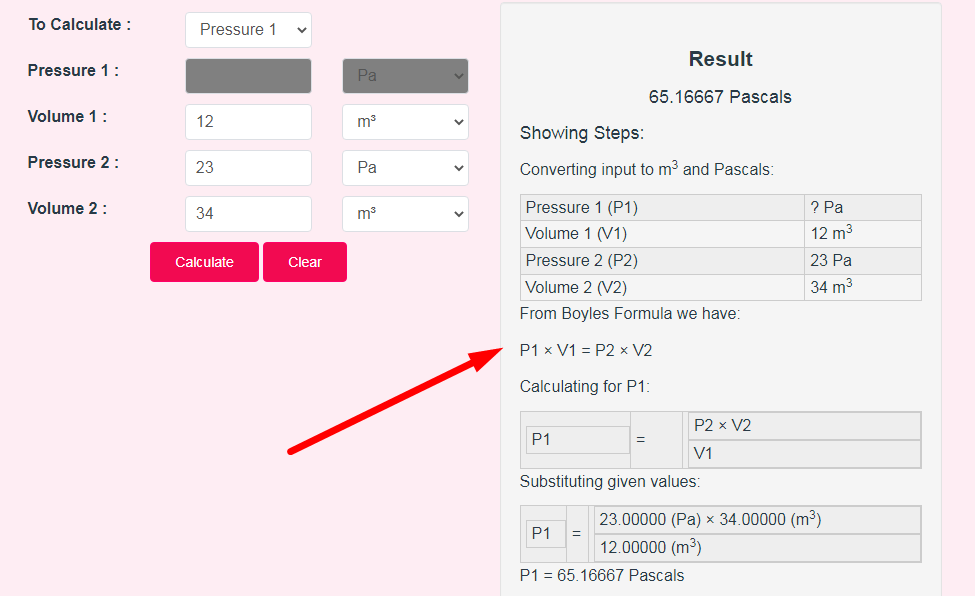
If you want to link to Boyles Law Calculator page, please use the codes provided below!
FAQs for Boyle's Law Calculator
What is a Boyle's Law Calculator?
A Boyle's Law Calculator is a tool used to calculate changes in the volume and pressure of a gas at constant temperature, based on Boyle's Law, which states that the pressure of a gas is inversely proportional to its volume when temperature is held constant.
How is Boyle's law used in everyday life?
He found that if you pressurize a gas, its volume contracts. If you decrease its pressure, its volume increases. You can observe a real-life application of Boyle's Law when you fill your bike tires with air. When you pump air into a tire, the gas molecules inside the tire get compressed and packed closer together.
What are the limitations of the Boyle's law?
This law cannot be used for low temperatures and high pressures. This law works for low pressures only. At high pressure, the temperatures and pressures are not constant but the value will be increased slightly because of the rising volume among molecules.
What is Boyle's law only valid for?
Answer: (a), Boyle's law is valid only for ideal gases. Q6. What is Boyle's law? Answer: Boyle's law depicts the relationship between the pressure, volume, and temperature of a gas.
What does Boyle's law calculate?
This empirical relation, formulated by the physicist Robert Boyle in 1662, states that the pressure (p) of a given quantity of gas varies inversely with its volume (v) at constant temperature; i.e., in equation form, pv = k, a constant. The relationship was also discovered by the French physicist Edme Mariotte (1676).